It has been a little more than a month since the FDA granted an EUA for our rapid antibody IgM and IgG blood test.
As expected, this announcement caused sales of our rapid blood test to skyrocket.
The increased business has provided us many opportunities to dialogue with customers and uncover questions about the Healgen antibody test and highlight where additional clarity is needed
Here is a quick review of what the antibody tests are looking for and how to read the results.
Depending on where a patient is on the Coronavirus timeline, their body will likely produce two different antibodies to counter the virus: IgM and IgG.
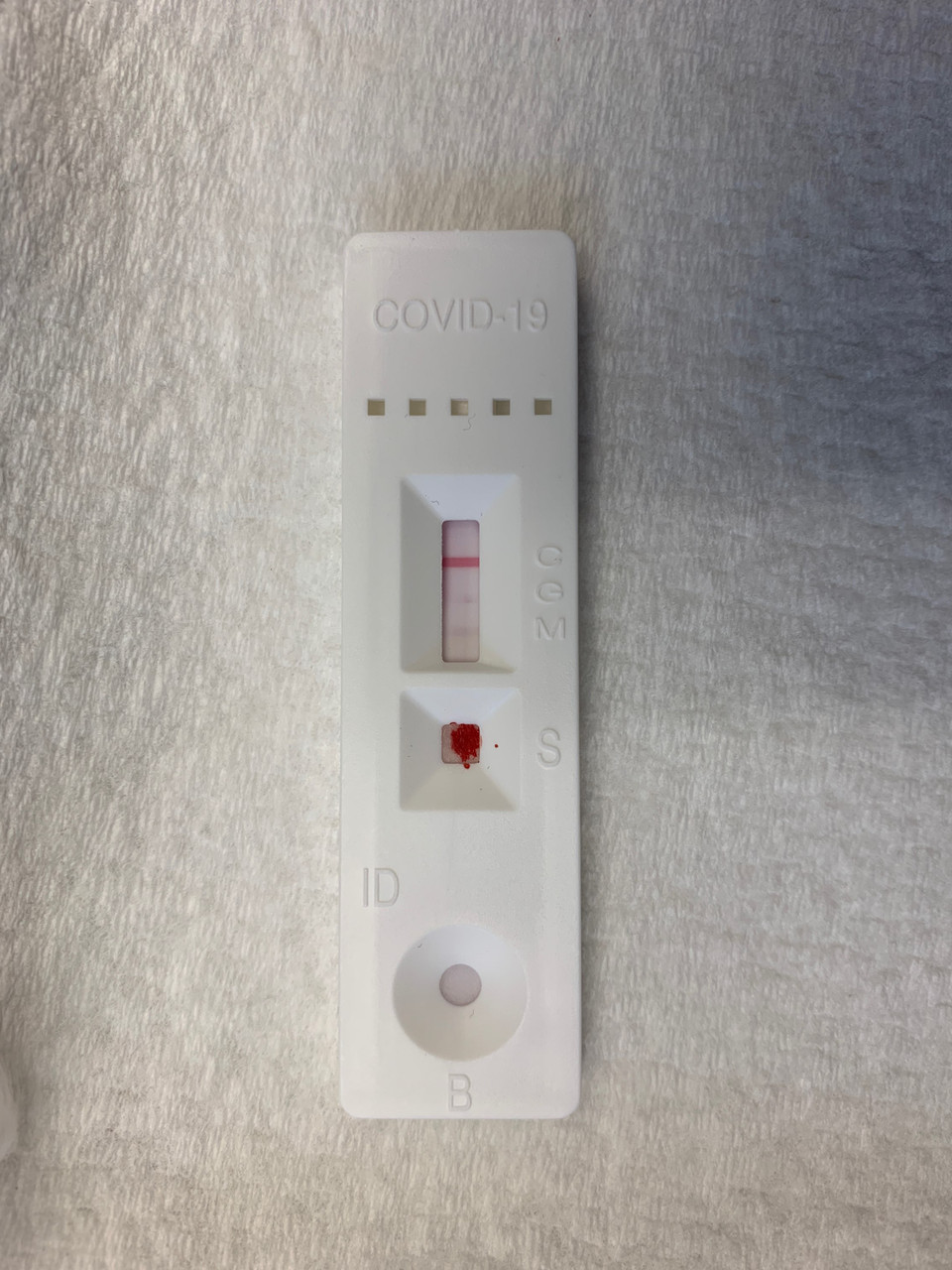
The test cassette contains three separate band lines which are treated to react and change colors when they detect the presence of a specific antibody.

A test may show a patient is completely negative (only the control line changes color, as below) or a combination of IgM positive/negative or IgG positive/negative, as seen in one of the three above.

Due to the sensitive biochemistry involved in these seemingly simple tests, results must be read within a specific window of about 5-10 minutes following the application of the blood sample and the buffer.
Results cannot be considered valid after fifteen minutes.
We recommend preparing the patient information in advance, such as writing their name, date and ID on a notecard, and taking a cell phone picture during the window to record the test results including the test cassette and the data card.
Should the control line fail to turn from blue to red, the test is invalid and the procedure needs to be repeated.

Here are the four most common questions we have been receiving about the Healgen Scientific rapid blood test.
Q: The test came back negative. Does that mean my employee/subject is allowed to come to work?
A: Because it takes about seven days for the body to product IgM antibodies, a second test should be done in one week’s time.
If both tests come back negative, then it is very likely this person does not have COVID-19
Q: The IgM and IgG bands are visible but faint. Does this mean the patient is nearly recovered or had only a mild case?
A: The Healgen Scientific antibody tests can detect the presence of these two prevalent antibodies, but are not designed to show the titer levels, or the volume of antibodies this patient has produced.
If the test lines are visible, the patient has tested positive.

Q: A patient tested positive for the IgM antibody. Should I send them home to quarantine?
A: Not without sending them for confirmation with a PCR test first.
Several times in the EUA document and repeated in the product literature, the FDA makes it clear they do not want antibody tests to be relied upon as a diagnostic test.
If a patient tests positive for IgM, they need to have a follow up PCR test to confirm they are currently infected before making the decision to isolate.
Q: Well, what then is the purpose of these antibody tests if they cannot answer this simple question?
A: It seems the FDA is most interested in using antibody tests as a data collection tool to help shape the knowledge of how people are responding to infection and to offer insight into the “million-dollar-question”: “Do antibodies provide short term or long term immunity from future COVID-19 infections?”
This is how the FDA closes the paragraph that addresses the question of serology tests:
“Serology tests can play a critical role in the fight against COVID-19 by helping healthcare professionals identify individuals who have antibodies to SARS-CoV-2 virus and have developed an adaptive immune response. In the future, this may potentially be used to help determine, together with other clinical data, whether these individuals may be less susceptible to infection.
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. In addition, these test results can aid in determining who may be eligible to donate a part of their blood called convalescent plasma, which may serve as a possible treatment for those who are seriously ill from COVID-19."