What is electroblotting?
Western blotting is a standard procedure used to analyze proteins. During this process, proteins are separated by their size using SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred from the gel to a sturdier membrane support that is probed with antibodies to detect specific proteins. The efficiency of the transfer step—also known as electroblotting—is crucial for successful Western blots.
How does protein transfer work?
Electroblotting is a two-step process. First, the polyacrylamide gel and membrane are laid on top of each other and flanked with other elements to generate a transfer stack, or “sandwich.” Next, an electrical current is applied across the sandwich to drive proteins out of the gel and onto the membrane.
What are the different protein transfer methods, and which is best?
To transfer proteins onto membranes, there are three main techniques—wet, semi-dry, and dry—that use different set-ups and lab equipment to accomplish the steps described above. Ultimately, the best method for you depends on your personal preference and several factors: time, cost, and experimental design. Here, we highlight the advantages and disadvantages of each method so that you can make an informed decision.
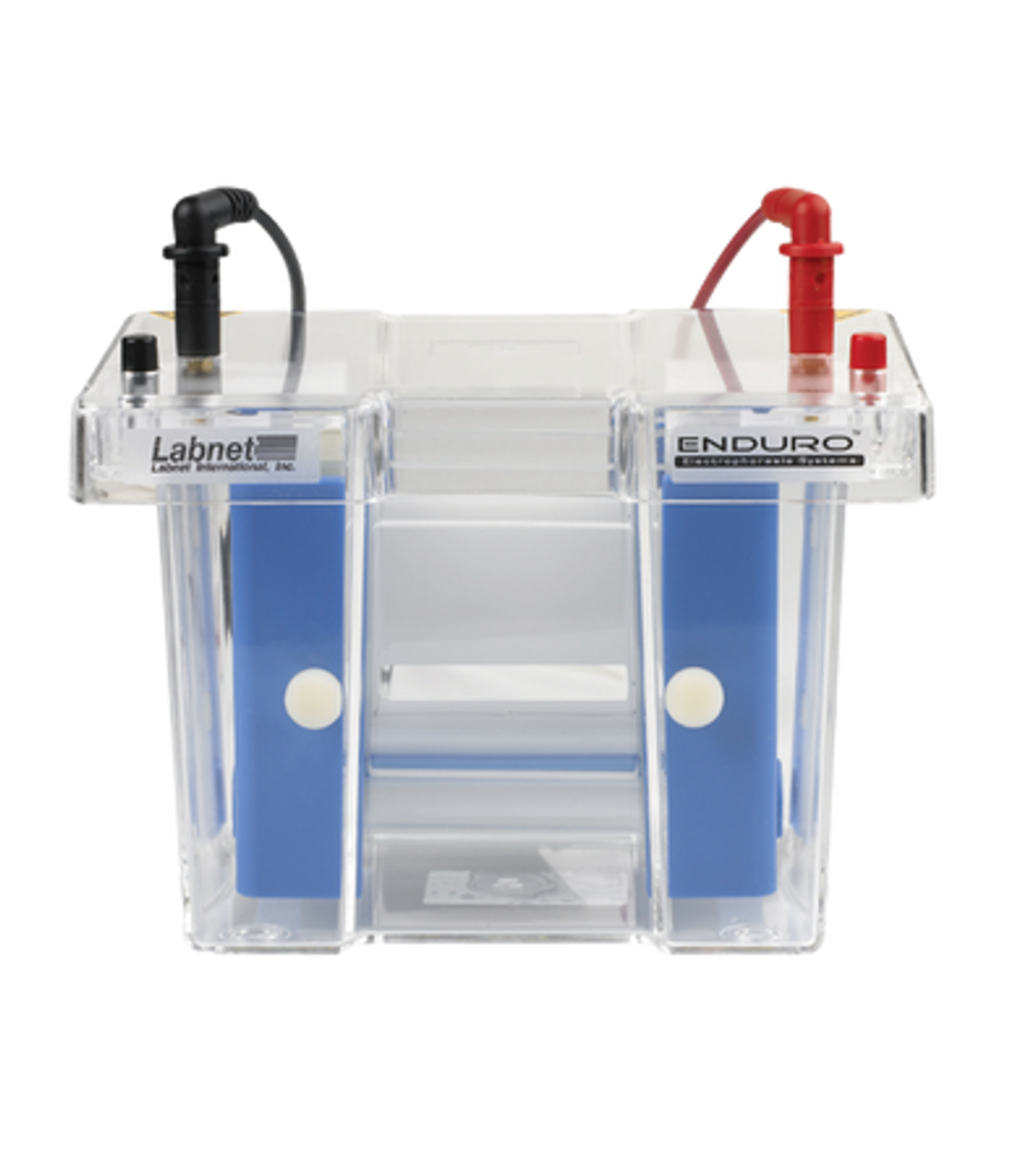
Wet transfers:
For this traditional transfer method, the gel-membrane sandwich is vertically submerged in a buffer-filled tank to facilitate current flow. Because the sandwich is prepared under buffer, air bubbles can form and disrupt transfer uniformity. When set up with sufficient care, however, wet electroblotting yields high transfer efficiencies for proteins of all sizes.
Another advantage of the wet transfer method is its versatility. Compatible with all polyacrylamide gels—homemade or precast—and membranes, wet electroblotting is relatively inexpensive and can be tailored to meet your needs.
The assembly and transfer steps of wet electroblotting are both time intensive, with runs lasting anywhere from an hour to overnight. Because it uses a large volume of current-conducting buffer, this technique generates excessive heat and requires a cooling mechanism to prevent band distortion.
Semi-dry transfers:
This method uses a decreased buffer volume to limit the flow of current to the gel-membrane interface. Instead of being submerged in a buffer-filled tank, the gel and membrane are placed between sheets of wet filter paper—these can be stacked to transfer several gels at once—that are in turn sandwiched between electrode plates.
Like wet transfers, the semi-dry method is customizable and compatible with all kinds of gels and membranes. However, semi-dry electroblotting has shorter transfer times that range from just ten minutes to an hour. Because it requires less buffer, this method is affordable and offers easy assembly.
With semi-dry electroblotting, the transfer efficiency depends on protein size—large proteins may not transfer well, and small proteins can run through the membrane unless the current is carefully controlled. Therefore, this method is not suitable for some experiments.
Dry transfers:
The fastest and simplest method, dry electroblotting is growing in popularity because of the time savings—assembly and transfer each take less than ten minutes. The gel and membrane are sandwiched between specially-formulated gel matrices that replace traditional buffers and tolerate high currents without generating excessive heat.
To limit band distortion, dry transfers use copper electrodes that don’t produce oxygen gas. Though initially low, transfer efficiencies are improving as the dry electroblotting technology advances.
Because dry electroblotting equipment is typically compatible with a single brand of polyacrylamide gels, versatility is limited. Dry transfers are also cost-prohibitive for many labs due to the price of purchasing commercial gels and single-use transfer stacks for each experiment.
Stellar Scientific currently offers small- and large-format lab equipment for wet electroblotting. We also carry semi-dry electroblotting equipment.